University Hospitals Research Published in NEJM Shows Minimally Invasive Procedure Saves Most Patients with Severe Vascular Disease from Amputation
April 02, 2023
New therapy helped 76 percent of patients at risk for major amputation keep their leg
Innovations in Cardiovascular Medicine & Surgery | Summer 2023
A study published in the March 30 issue of the New England Journal of Medicine has shown that there may finally be an alternative to amputation for patients suffering from chronic limb-threatening ischemia (CLTI), the most severe form of peripheral artery disease. This study, co-led by University Hospitals (UH) Harrington Heart & Vascular Institute, could lead to the first FDA approval of a therapy giving thousands of patients hope for an alternative to limb loss.
Therapy Saves Most Patients from Amputation
The PROMISE II U.S. pivotal clinical trial found that minimally invasive LimFlow therapy enabled most patients to avoid amputation and experience wound healing. The procedure is designed to bypass blocked arteries in the leg and rush blood back into the foot through the veins.
 Mehdi Shishehbor, DO, MPH, PhD
Mehdi Shishehbor, DO, MPH, PhDMehdi Shishehbor, DO, MPH, PhD, President of UH Harrington Heart & Vascular Institute, and Angela and James Hambrick Chair in Innovation, as well as lead author and co-principal investigator of the study, said, “LimFlow is a unique and novel alternative to major amputation, providing hope where previously there has been none. The results from this study are excellent and it’s very clear that LimFlow is a powerful tool for avoiding amputation.”
University Hospitals Harrington Heart & Vascular Institute is the only program in Ohio to participate in the PROMISE II U.S. pivotal clinical trial with LimFlow therapy.
In the paper entitled “Transcatheter Arterialization of the Deep Veins in Chronic Limb-Threatening Ischemia: The PROMISE II Multicenter Prospective Study,” investigators evaluated 105 CLTI patients who were treated with transcatheter arterialization of the deep veins (TADV) using the LimFlow therapy. All patients were facing inevitable amputation before the procedure. At six months post-procedure, 76 percent of patients were able to keep their leg (also called limb salvage). Within the same time period, 76 percent of patients had completely healed or healing wounds. Freedom from all-cause mortality was 87 percent at six months.
In the study, the median age of participants was 70 years, with a range of 38 to 89 years old. The study was designed to include diversity, with 31 percent of patients being female and 43 percent being Black, Hispanic, or Latino. The inclusion of a large number of non-white CLTI patients was important because they are disproportionally at risk of amputation compared to white patients.
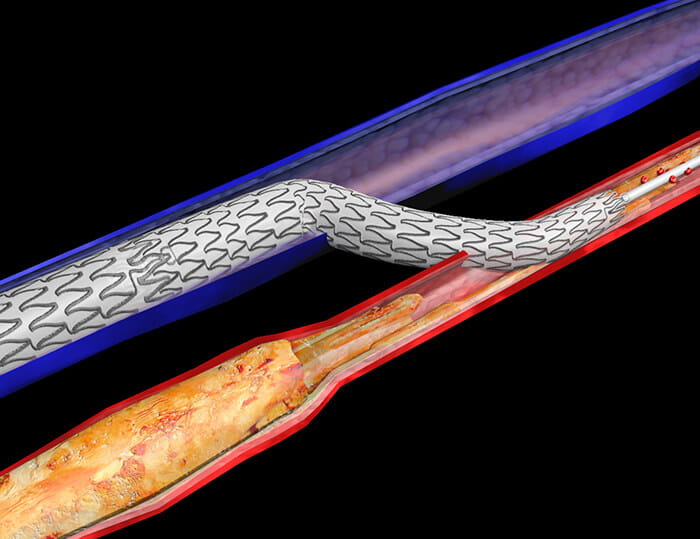 LimFlow crossing stent.
LimFlow crossing stent.Amputation: An Option with too Many Problems
CLTI represents the end stage of peripheral artery disease, when poor circulation in the limb causes symptoms including numbness, and absent or diminished pulse in the feet or legs. Open sores, skin infections or ulcers that will not heal can cause gangrene and extreme pain. It is estimated that two million Americans may be living with CLTI and, for the many with comorbidities like diabetes, treatments such as bypass surgery or endovascular revascularization may not be feasible. For these patients with no other options, the limb begins to turn black and die, and amputation may become imminent.
“Although this disease has existed for decades, research and innovations in treatment options have been lacking,” said Dr. Shishehbor. “We want to improve our patients’ lives, so we don’t take amputation lightly. While it can alleviate some issues related to disease, it brings its own challenges which can include disability, emotional distress, and even death.”
The Amputee Coalition of America estimates more than 500 patients undergo an amputation every day. Around 30 percent of amputees experience depression or anxiety. Amputees can have “phantom pain” in the missing limb that causes stabbing, burning or shooting sensations. Nearly half of all patients with vascular disease will die within five years after amputation, which is higher than the five-year mortality rates for breast cancer, colon cancer and prostate cancer.
“Approximately 185,000 amputations occur in the United States each year and a staggering 3.6 million people will be living with limb loss by 2050,” said Dr. Daniel Simon, cardiologist and President, Academic & External Affairs and Chief Scientific Officer, Ernie and Patti Novak Distinguished Chair in Health Care Leadership, University Hospitals, and Professor of Medicine and Senior Associate Dean for Academic Affairs, Case Western Reserve University School of Medicine. “These results are potentially a game changer for these patients and their families.”
Living Proof
Linda McClain of Glouster, Ohio enjoys going to the salon, grabbing lunch with her friends and line dancing. But in 2018, a diabetic ulcer appeared on her toe and wouldn’t go away. It caused pain and limited her activities. After several failed procedures at another hospital, circulation to her left foot couldn’t be restored.
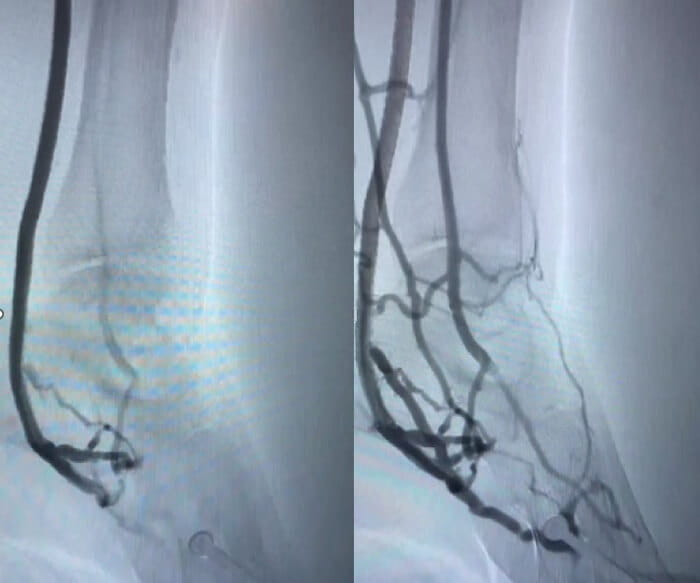 Scans of patient before and after LimFlow.
Scans of patient before and after LimFlow.“It was really hard when the toes started to turn black. There wasn’t any blood flow to my foot. It was cold all the time and I just had a lot of pain,” said McClain.
She was headed toward a below-the-knee amputation. Instead, in April of 2020 she found Dr. Shishehbor. McClain was approved to participate in the PROMISE II clinical trial and in June of that year, underwent the LimFlow procedure at UH Cleveland Medical Center. Her toes were too far gone and needed to be removed, but she was able to keep her leg. Now, at 76 years old, she wears an orthotic and walks on her own with no pain. She credits Dr. Shishehbor and the LimFlow procedure for saving her left foot and leg.
“My pain is greatly decreased, and I have more independence,” she said. “Losing my leg would have been detrimental to the life I want to live. I’m grateful to Dr. Shishehbor for performing this procedure and improving my life.”
“These patients are literally called ‘no-option’ patients. No option means no hope,” said Dr. Shishehbor. “With this study, we are providing hope for a safe and effective alternative to amputation and a better life for thousands of patients. Once approved by the FDA, we look forward to this procedure being widely available to patients who so desperately need it.”


