Collaboration Between University Hospitals Harrington Heart & Vascular Institute and Yale University Studies Pharmacologic Treatment for Obstructive Sleep Apnea
November 08, 2022
Innovations in Cardiovascular Medicine & Surgery | Fall 2022
A National Institutes of Health (NIH) RO1 grant is exploring a promising pharmacologic sodium-glucose cotransporter-2 (SGLT2) inhibitor to treat obstructive sleep apnea (OSA). Ian Neeland, MD, FAHA, FACC, Director of Cardiovascular Prevention and Co-Director of the Center for Integrated and Novel Approaches in Vascular-Metabolic Disease within University Hospitals Harrington Heart & Vascular Institute, is a Co-Principal Investigator, along with Henry Yaggi, MD, MPH, Director of the Yale Centers for Sleep Medicine.
 Ian Neeland, MD
Ian Neeland, MD“The grant was born out of an observation that we made in previous trials that patients with diabetes who were treated with a medication from the SGLT2 inhibitor class displayed a 50 percent reduction in new-onset obstructive sleep apnea versus patients who were treated with placebo,” says Dr. Neeland.
According to the American Medical Association (AMA), an estimated 30 million people in the U.S. have OSA, but roughly only six million have been formally diagnosed. The pervasive sleep disorder is linked to obesity, diabetes, insulin resistance, pulmonary hypertension, and elevated risk of cardiovascular disease and stroke. “There is also a multifactorial impact on quality of life, contributing to daytime sleepiness, diminished work efficiency and depression,” says Dr. Neeland.
While the gold standard treatment has been continuous positive airway pressure (CPAP), patient compliance is inconsistent. “Only half of people prescribed these machines tolerate them long term,” says Dr. Neeland. He adds that surgery may provide relief for appropriately selected patients. The need for novel treatments to address OSA has stirred interest in the potential of SGLT2 inhibitors, initially developed for Type 2 diabetes and approved to treat hyperglycemia by preventing the kidneys from resorbing glucose back into the blood.
The RO1 grant supports a randomized, placebo-controlled clinical trial at University Hospitals and Yale New Haven Health. Each site will recruit 82 individuals with moderate to severe OSA and a body mass index (BMI) of 25-40. Participants will be randomized to Ertugliflozin 15 mg once daily or matching placebo and standard clinical care over six months. The timeline for the study is five years.
Researchers will utilize Nox Medical’s sophisticated in-home sleep apnea testing, which can measure several parameters of sleep patterns and oxygenation. Study participants will wear a biosensing wrist device that gathers data on sleep duration and efficiency, receive 24-hour home blood pressure monitoring and complete sleep questionnaires. “We will hopefully answer the question — at least preliminarily — whether a SGLT2 inhibitor can treat sleep apnea and better understand the underlying mechanisms of the therapy,” says Dr. Neeland.
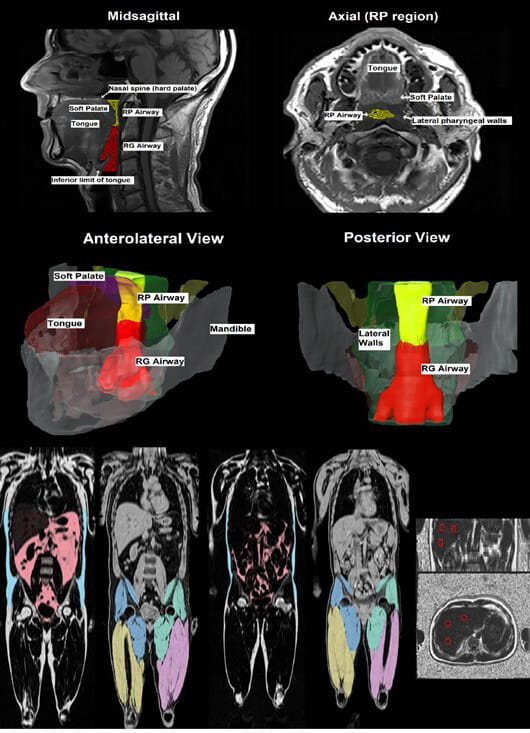 Examples of segmentation of airway structures including the tongue, retropharyngeal airway, soft palate, and lateral walls. Examples of segmentation of visceral AT (red), subcutaneous AT (blue), and thigh muscles in obese (left) and lean (right) participants. Example of liver fat fraction (in coronal and axial views).
Examples of segmentation of airway structures including the tongue, retropharyngeal airway, soft palate, and lateral walls. Examples of segmentation of visceral AT (red), subcutaneous AT (blue), and thigh muscles in obese (left) and lean (right) participants. Example of liver fat fraction (in coronal and axial views).As part of the investigation, researchers are conducting detailed assessments and imaging to evaluate the impact of the medication on anatomic and physiologic traits in addition to clinical outcomes. Dr. Neeland explains that half of OSA is related to excess fat in the tongue and neck blocking the airway, while physiologic changes impact the other half.
“We are fortunate to have Dr. Rich Schwab, a world-renowned sleep expert at the University of Pennsylvania, collecting exquisitely detailed MRI anatomical imaging,” says Dr. Neeland. Researchers will obtain measurements of abdominal visceral and subcutaneous adipose tissue, lower body (hips and buttocks) adipose tissue, muscle fat infiltration, liver fat, thigh muscle lean tissue volume, and total body fat and lean tissue volumes.
The team will also be deriving state-of-the-art phenotyping of the anatomic and non-anatomic traits of OSA using routine polysomnography pioneered by Scott A. Sands, PhD, a consultant on the study who is based at Brigham and Women's Hospital in Boston. “We are collaborating with people who are leaders in the field of sleep medicine to ensure that this is a rigorous and mechanistic trial of OSA that will be generalizable to large populations,” says Dr. Neeland. “If the results are positive, we will hopefully spur additional research into the potentially first medical therapy for obstructive sleep apnea.”
For more information, contact Dr. Neeland at Ian.Neeland@UHhospitals.org.
Contributing Expert:
Ian Neeland, MD, FAHA, FACC
Director, Cardiovascular Prevention, and
Co-Director, Center for Integrated and Novel Approaches in Vascular-Metabolic Disease
University Hospitals Harrington Heart & Vascular Institute
Associate Professor
Case Western Reserve University School of Medicine


