New Immunotherapy Center at UH Seidman Cancer Center Sets the Stage for Solutions
February 10, 2021
Center offers dozens of clinical trials, including soon a rare all-human protein CAR T trial for patients with certain hematologic malignancies
Innovations in Cancer | Winter 2021
A $10 million gift from Kimberly and Joseph Wesley to establish the Wesley Center for Immunotherapy at UH Seidman Cancer Center will dramatically expand the footprint and capabilities of UH’s Cell Therapy Laboratory, which generates CAR T, NK cell and other cell- based therapies with faster turnaround times for patients than are available commercially. Already, UH has 61 immunotherapy trials open, including 17 immunotherapy-centered clinical trials originating from the UH cell therapy facility. The expansion of the facility will allow these numbers to grow, supporting new CAR T trials and other promising immunotherapy research.
 Ted Teknos, MD
Ted Teknos, MD”We are tremendously expanding our space, with about 3,600 additional square feet of lab space, which will be used to create novel cell therapies and treatments for our patients,” says Ted Teknos, MD, President and Scientific Director of UH Seidman Cancer Center and Professor of Otolaryngology and Head & Neck Surgery at Case Western Reserve University School of Medicine. “The new space will also include three new clean rooms, which will double our capabilities for providing natural killer cell and other genetically modified cell therapies for our patients.
 Paolo Caimi, MD
Paolo Caimi, MD”While planning and construction of the new facility are ongoing, however, the hematologists and oncologists at UH Seidman are continuing their work pursuing novel immunotherapy research questions and offering their cancer patients the latest cell-based clinical trials. In fact, UH Seidman Cancer Center will soon launch a new CAR T trial that uses fully human protein – one just a handful of sites nationwide to offer this option to patients.
“The antibody part of the antigen receptor that we’re putting on the surface of the T cell is a fully human sequence,” says oncologist Paolo Caimi, MD, who is leading this clinical trial at UH Seidman Cancer Center. Dr. Caimi is also Associate Professor of Medicine at the School of Medicine. “In theory, it may have fewer adverse events, so be slightly safer, while still attacking the same target. The biggest question is whether by not having a foreign component, it may be a little bit more effective in instigations in the signaling. Human to human should connect a little bit better than human to mouse, and it may not generate, the type of reactions that we are used to seeing with a regular CAR Ts. So that's what we're going to explore.”
Over the course of 18 months to two years, Dr. Caimi hopes to enroll between 18 and 36 patients in the Phase I clinical trial.
“We're going to do one study that has two parallel cohorts,” he explains. “Patients with non-Hodgkin's lymphoma and chronic lymphocytic leukemia will be in one group. For the other cohort, we'll be doing acute lymphoblastic leukemia, which is an aggressive type of cancer, but also has the CD19 on the surface. We'll do a pilot cohort to test it in both groups of patients.”
If the fully human CAR T-cell therapy were to be successful in ALL patients, it would greatly expand their current options, Dr. Caimi says.
“There's only one approved CAR T product, and that CAR T product is good for leukemia in kids,” he says. “It’s not approved for leukemia in adults. We're really just trying to add another option for adults with acute lymphoblastic leukemia. The problem is that people with ALL will have more side effects from CAR T. So hopefully this product will be safer and it can be done in a more active fashion and we’ll be able to treat adults advanced disease. Because right now, the options are few.”
Beyond hematologic malignancies, oncologists at UH Seidman Cancer Center are also exploring the potential of CAR T-cell therapies to attack solid tumors. Jordan Winter, MD, UH Seidman’s Director of Surgical Services and Division Chief of Surgical Oncology at UH Cleveland Medical Center, has a special interest in pancreatic cancer. He’s beginning what he believes may be a long journey to bring CAR T-cell therapy to fruition for these patients, beginning with targeting the protein mesothelin. Dr. Winter is also Professor of Surgery and Biochemistry at the School of Medicine.
The significant cellular differences between hematologic malignancies and solid tumors make this work complex, he says.
“CAR T right now is approved for just a few certain lymphomas,” Dr. Winter says. “The tumors that it's approved for all express a protein or an antigen in common. Every cell in that patient's cancer expresses that antigen. So if you design a T cell to target that antigen, it will uniformly eradicate it. There's no such equivalent antigen in pancreas cancer. Mesothelin is expressed in in about half of pancreatic cancers. But in a given pancreas cancer, it's only expressed in about one third of those cells.”
However, Dr. Winter adds, there is a path there to help patients. The expanded capabilities of the Cellular Therapy Laboratory will allow UH oncologists like himself to target multiple antigens at once, developing a complex CAR T product that matches the complexity of the tumor.
“We're not just targeting mesothelin, but also targeting other antigens to increase our total kill rates, expanding it beyond just the third of cancer cells,” he says. “Is it going to take doing a CAR T with one additional antigen or two or 20? We don’t know that yet.”
As a beginning, Dr. Winter is conducting in vitro and in vivo experiments to measure the differential ability of genetically engineered CAR T cells to kill mesothelin-positive and mesothelin-negative pancreatic cancer cells. If this work is successful, he says he hopes to offer a clinical trial to patients within a year.
“We'll go into a clinical trial and we will look for patients that have mesothelin-positive tumors, and we'll do the study,” he says. ”But at the same time, we'll want to develop, strategies to make the CAR Ts more effective and to increase the number of antigens that the CAR Ts will target.”
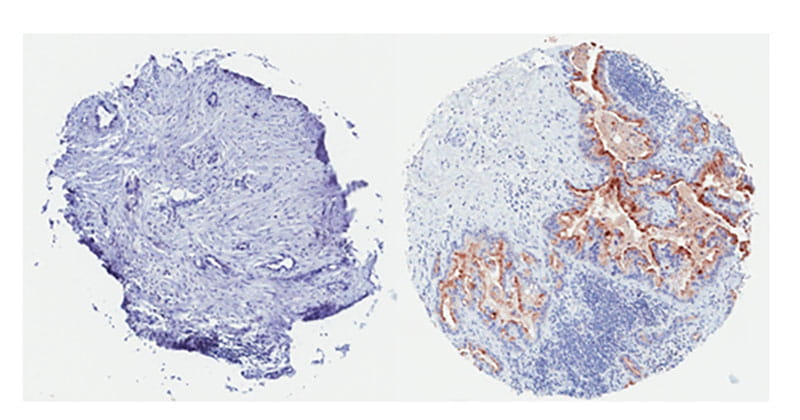 (Left) This pancreatic tumor is mesothelin-negative. (Right) This pancreatic tumor is widely positive for mesothelin expression and it is expected would respond well to mesothelin-directed CAR-T cell therapy.
(Left) This pancreatic tumor is mesothelin-negative. (Right) This pancreatic tumor is widely positive for mesothelin expression and it is expected would respond well to mesothelin-directed CAR-T cell therapy.Quintin Pan, PhD, UH Seidman’s Deputy Director of Research and Professor of Otolaryngology and Head & Neck Surgery at the School of Medicine, is also looking to target mesothelin in his immunotherapy research. He is conducting similar in vitro and in vivo experiments to gauge CAR T-cells’ ability to kill head and neck tumors.
“The hope is that we can generate very compelling pre-clinical data, both in vitro, as well as in animal models,” he says. “We're going to test cells that have high or low mesothelin expression. If this engineered T cells platform works as designed, then we should get selective ablation of the cancer cells with high mesothelin. Once we have accumulated a portfolio of pre-clinical data in a number of tumor types, then we can begin to plan to transition this technology to a clinical trial.”
Like pancreatic tumors, not all cells in mesothelin-positive head and neck cancers express high levels of mesothelin, complicating the CAR T approach. However, Dr. Pan and his UH colleagues are undaunted. With the new capabilities of the Wesley Center for Immunotherapy at UH Seidman Cancer Center, they say, the stage is set to find solutions.
“This adds an additional layer of complexity as we begin to better understand how we incorporate CAR T cell therapy for solid cancer patients,” Dr. Pan says. I suspect we're going to have to use a combination of different strategies and perhaps even a combination of CAR T cells. Fortunately, we have just the facility at UH for this kind of work."


