“ZeBRa” Stent in Development by UH Expert Poised to Meet Unmet Clinical Need
June 09, 2019
A better option for neonatal coarctation of the aorta
Innovations in Cardiovascular Medicine & Surgery - Summer | 2019
When it comes to treating neonatal coarctation of the aorta – one of the most common forms of congenital heart disease, there are currently few good options.
“Surgical repair continues to have an associated mortality rate of 2 percent, a recurrence rate of up to 18 percent and other considerable morbidity,” says Martin Bocks, MD, Director of Pediatric Interventional Cardiology at University Hospitals Rainbow Babies & Children’s Hospital; and Associate Professor of Pediatrics, Case Western Reserve University School of Medicine.
 Martin Bocks, MD
Martin Bocks, MDCurrent interventional approaches also have drawbacks, Dr. Bocks says.
“Balloon angioplasty is often of limited effectiveness and is associated with significant vessel recoil, vessel injury, high coarctation recurrence rate and late pseudoaneurysm formation,” he says.
Endovascular stenting with bare metal stents does not have these drawbacks, but there is currently no ideal bare metal stent option for neonates. Stents that are small enough to pass through the femoral arteries of infants and young children can’t be dilated later to accommodate the growth of the child.
“This rigid, fixed-diameter stent would significantly limit the growth of the aorta as the child ages if other interventions were not performed,” Dr. Bocks says. “For this reason, infants and young children with coarctation of the aorta represent a patient population without an optimal, safe and effective therapy.”
To address this challenge, Dr. Bocks has been working with a company called PediaStent to develop a novel bioresorbable zinc alloy stent for congenital heart disease patients. PediaStent was one of two companies in the U.S. to receive a $3.4 million Small Business Innovation Research (SBIR) grant from the National Institutes of Health to develop a stent specifically designed for neonatal coarctation of the aorta. After promising results in Phase I studies, the goal at the end of Phase II is to have a stent ready for a clinical trial in humans.
“This promising effort in research and development highlights the extraordinary expertise at University Hospitals in treating congenital heart disease, including even our growing adult congenital program,” says Marco A. Costa, MD, PhD, MBA, President of the UH Harrington Heart & Vascular Institute; and Professor of Medicine, Case Western Reserve University School of Medicine. ”It’s a prime example of how technology can enable our vision to be the most trusted, integrated academic heart and vascular institute that anchors a healthy community and defines the future of medicine.”
At UH, the congenital heart disease team includes more than 20 clinicians, including Dr. Bocks, Christopher Snyder, MD, Division Chief of Pediatric Cardiology, Eric J. Devaney, MD, Chief of Pediatric Cardiothoracic Surgery, and Pradeepkumar Charla, MD, MS, Director of the Adult Congenital Heart Disease Program, among others.
The bioresorbable stent under development is dubbed ZeBRa (Zinc BioResorbable Stent). According to Dr. Bocks, that name seems to fit the nature of the project.
“The name ZeBRa highlights the novelty of our technology,” he says. “But it’s also a fitting name for the primary indication of treating rare congenital heart conditions, for which dedicated medical devices are rarely developed.”
The plan for treating an infant with coarctation of the aorta would be to implant the ZeBRa stent in the coarctation segment of the aorta during the newborn period, Dr. Bocks says.
“The stent will provide complete relief of obstruction and will allow time for the aortic wall to model to an appropriate diameter matching that of the stent, after which the stent will degrade,” he says. “Many infants will likely need additional stenting with larger ZeBRa stents as complete remodeling might not occur for several years. However, this approach will obviate the need for higher-risk interventions, such as surgery or purposefully trying to fracture a rigid stent with an angioplasty balloon to ‘unzip’ it.”
According to Dr. Bocks, the ZeBRa stent has the potential to be a game-changer in treating neonatal coarctation.
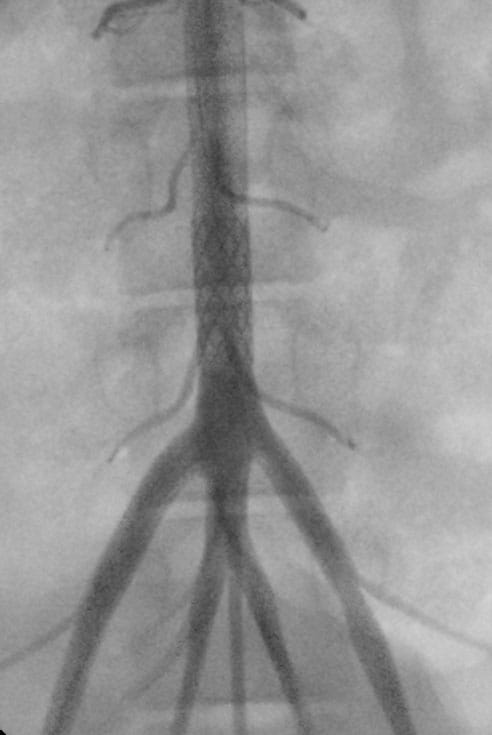 ZeBRa Stent implanted in the aorta in a preclinical model.
ZeBRa Stent implanted in the aorta in a preclinical model.“The stents have excellent radial force, degrade in the desired amount of time and have a very low crossing profile, which means we can get them into our young patients despite their femoral arteries being quite small,” he says.
Talks are under way with the U.S. Food and Drug Administration to complete the steps necessary to gain approval for use in the U.S.
In the meantime, Dr. Bocks says the ZeBRa stent project provides an instructive roadmap for effective collaboration between an academic medical center and a small medical device company, with the support of federal funding.
“I am proud of what we’ve accomplished so far and am looking forward to the day that we can offer new devices to some of our highest-risk congenital heart disease patients,” Dr. Bocks says.
For more information about this project, please email Martin.Bocks@UHhospitals.org.


