University Hospitals Cleveland Medical Center is Northeast Ohio’s Only Site Offering Remedē® System for Central Sleep Apnea
February 23, 2023
Innovations in Pulmonology & Sleep Medicine | Winter 2023
University Hospitals Cleveland Medical Center is Northeast Ohio’s only location offering implantation of the U.S. Food and Drug Administration (FDA)-approved remedē® System, a breakthrough device designed for the treatment of moderate to severe central sleep apnea (CSA).
 U.S. Food and Drug Administration (FDA)-approved remedē® System.
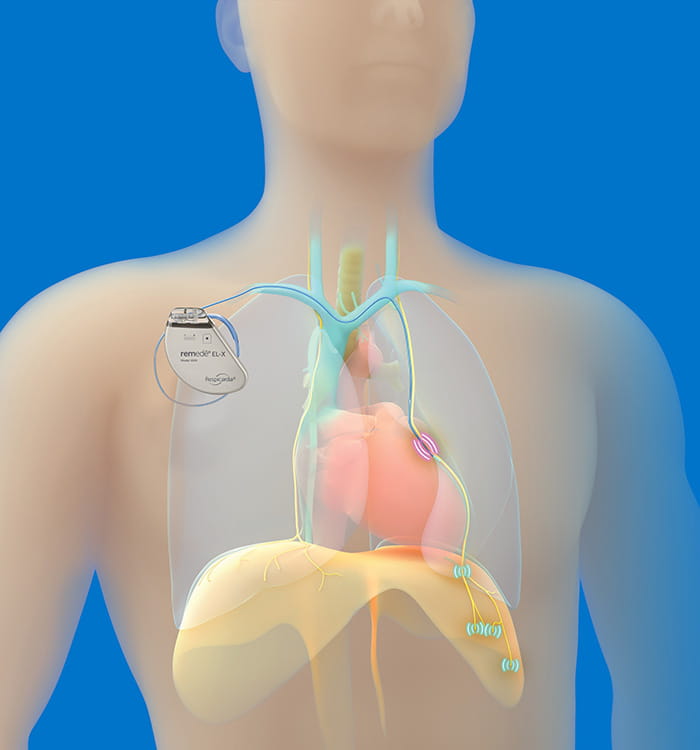
U.S. Food and Drug Administration (FDA)-approved remedē® System.“The remedē system is a fully implantable transvenous phrenic nerve stimulation device that sends signals to the diaphragm,” says Susheel Patil, MD, PhD, chief of Sleep Medicine at University Hospitals Cleveland Medical Center. “The phrenic nerve is responsible for signaling the diaphragm to contract, enabling inhalation.”
Offering the remedē system involves a collaboration between the UH Sleep Medicine Program and the UH Harrington Heart & Vascular Institute team because placement involves a minimally invasive outpatient procedure.
 Susheel Patil, MD, PhD
Susheel Patil, MD, PhD“One of the nice aspects about offering this novel technology at UH is that it highlights the close-knit integration between our different service lines,” says Dr. Patil. “After the initial screening, our colleague, Dr. Shashank Jain, will do the implantation, and then sleep medicine will manage the patients’ progress and make necessary adjustments to the device settings.”
Shashank Jain, MD, a cardiac electrophysiologist in UH Harrington Heart & Vascular Institute, performs the remedē procedures. Dr. Jain trained on this procedure with Ralph Augostini, MD, FACC, an electrophysiologist at Ohio Health in Columbus, who participated in the pivotal trial, the first multicenter randomized investigation of the safety and efficacy of the remedē system.
 Shashank Jain, MD
Shashank Jain, MD“The device is placed using fluoroscopic imaging guidance. It is similar to a traditional pacemaker in terms of incision but is placed on the right side, whereas cardiac devices like pacemakers and defibrillators are left-sided,” says Dr. Jain. “Additionally, the leads we use are placed in a vein on the outside of the heart, whereas traditional cardiac leads are placed directly into the heart’s main chambers.”
Improved Oxygenation and Quality of Life
Clinical trial shows that the innovative therapy monitors and stabilizes breathing patterns and reduces the number of sleep apnea events. Documented improvements of CSA include:1
- 87 percent of patients had a reduction in apnea-hypopnea index, an indication of CSA severity
- 79 percent of patients had an improvement in quality of life
- 91 percent of patients were free from serious adverse events related to the implant procedure, the device, or delivered therapy at 12 months
Understanding Central Sleep Apnea
Unlike obstructive sleep apnea (OSA), which occurs when the physical structures of the airway relax, narrow or close, CSA is caused by the brain failing to signal the diaphragm to regulate breathing consistently.
Dr. Patil defines CSA as the absence of airflow in the absence of effort and OSA as the absence of airflow in the presence of effort. “There is a tightly integrated system between the brain, lungs and heart,” he says. “When the organs are not communicating with each other properly, it can lead to periods where the brain briefly stops sending signals to the breathing muscles.”
Individuals affected by CSA experience inconsistent breathing patterns and repeated arousals from sleep. Patients with CSA are at risk for drops in blood oxygen levels and increased cardiac stress response. In addition to more serious complications, CSA can diminish quality of life, including problems with chronic fatigue, daytime sleepiness or memory.
The majority of patients with CSA are experiencing heart failure or atrial fibrillation, and their symptoms can overlap with co-existing health challenges. Candidates for remedē must first complete a sleep study to determine the frequency and severity of their disordered breathing. To be considered a candidate for implantation, patients must experience 20 or more apnea or hypopnea events per hour, and at least 50 percent of all apneas must be diagnosed as central versus obstructive.
Appropriate Patient Selection
Currently, University Hospitals Cleveland Medical Center is screening patients who may be appropriate candidates for remedē. “Central sleep apnea is far less common than obstructive sleep apnea,” says Dr. Patil. “The condition might represent 10 percent of the apnea we treat, but we are excited to offer an important therapy for patients who are not able to tolerate or find improvement from traditional CSA management.”
For more information, contact Dr. Patil at Susheel.Patil@UHhospitals.org.
1.Costanzo M, et al. Transvenous neurostimulation for central sleep apnea: a randomized controlled trial. The Lancet. 2016; 388: 974–82.
Contributing Experts:
Susheel Patil, MD, PhD
Chief, Sleep Medicine
University Hospitals Cleveland Medical Center
Clinical Associate Professor
Case Western Reserve University School of Medicine
Shashank Jain, MD, FACC
Clinical Cardiac Electrophysiologist
University Hospitals Harrington Heart & Vascular Institute
Clinical Assistant Professor
Case Western Reserve University School of Medicine
Tags:


