New Steps Protect Clinicians Exposed to Hepatitis C
January 17, 2021
“Test-and-treat” strategy takes advantage effective, well-tolerated antiviral treatments
Innovations in Digestive Health | Winter 2021
 Stanley Cohen, MD
Stanley Cohen, MDIn the era of COVID-19, many other infectious diseases have been overlooked. However, a viral infection — one without a vaccine — has received recent attention from the Centers for Disease Control and Prevention (CDC), the American Association for the Study of Liver Diseases (AASLD) and the Infectious Disease Society of America (IDSA).
Hepatitis C (HCV), a significant cause of morbidity and mortality, is on the rise. To ensure patients and exposed healthcare professionals receive immediate care, University Hospitals has updated its testing and treatment guidelines to conform with the latest CDC guidance.
University Hospitals has brought innovative research and treatment options to HCV patients for the past two decades. The University Hospitals Digestive Health Institute Liver Diseases Center has one of the largest HCV clinics in the region, with more than 1,000 patients treated and cured within the last several years.
Unfortunately, the numbers are moving in the wrong direction. New (acute) HCV infections have increased from 29,700 cases in 2013 to 50,300 in 2018 according to CDC reports. The agency attributes these numbers to a rise in IV drug use.
With escalating HCV infection comes increased risk of occupational exposure. While the risk of contracting HCV infection from a patient is low for healthcare professionals — about 0.2% in cases with percutaneous exposure — the CDC recommends a “don’t wait” approach to testing and treatment.
TREATMENT AND TESTING: WHAT'S CHANGED?
“In the past, if a physician got a needle stick injury and became infected with HCV, we would wait to see if it spontaneously resolved,” says Stanley Martin Cohen, MD, FAASLD, FACG, Medical Director, Hepatology at UH Digestive Health Institute, and Professor of Medicine at Case Western Reserve University School of Medicine. “Now, if you test positive, you get treated early.”
The CDC, AASLD and IDSA recommend a “test-and-treat” strategy for people with acute HCV infection on initial diagnosis. Not only does waiting increase risk of transmission (depending on HCV-RNA level), it also creates undue anxiety for the exposed clinician. Because today’s antiviral treatments are simple, effective and very well tolerated, there’s simply no reason to wait.
As an example of the effectiveness of available HCV treatments, an open-label, two-cohort, phase 1 clinical trial that investigated an eight-week regimen of ledipasvir/sofosbuvir for acute HCV infection in men reported a 100% sustained virologic response.1 A review of HCV treatment with ledipasvir/sofosbuvir reported a high sustained virologic response in both adults and adolescents.2 Vosevi, an antiviral medication approved in 2017, demonstrated a 96-97% success rate after 12 weeks of treatment.3
“HCV treatment is much easier and more effective than 20 years ago,” Dr. Cohen says. “With little to no side effects, we can cure over 95 percent of infected patients.”
THE TEST-AND-TREAT ALGORITHM
The CDC recommends testing the source patient within 48 hours after exposure. The preferred test: HCV-RNA. If the source patient has undetectable HCV-RNA (even if the HCV antibody is positive), they do not have active infection and are not considered infectious to the healthcare professional.
After a needle stick injury or other exposure, the professional should get tested for both HCV antibody and HCV-RNA within 48 hours of exposure. A positive result to either test indicates previous exposure and/or infection. An initial antibody-positive and HCV-RNA-negative result indicates the professional either cleared the infection or received previous treatment.
If the source patient is HCV-RNA positive, and/or if the patient shows evidence of active HCV-RNA, the exposed healthcare professional will need follow-up HCV-RNA tests to assess infection. If follow-up tests show positive HCV-RNA after exposure, the professional should receive treatment as soon as possible.
It’s also important to take HIV infection risk into account. “HIV transmission can also occur percutaneously or mucocutaneously,” Dr. Cohen says. “Appropriate evaluation of HIV transmission must also be considered for exposed healthcare professionals.”
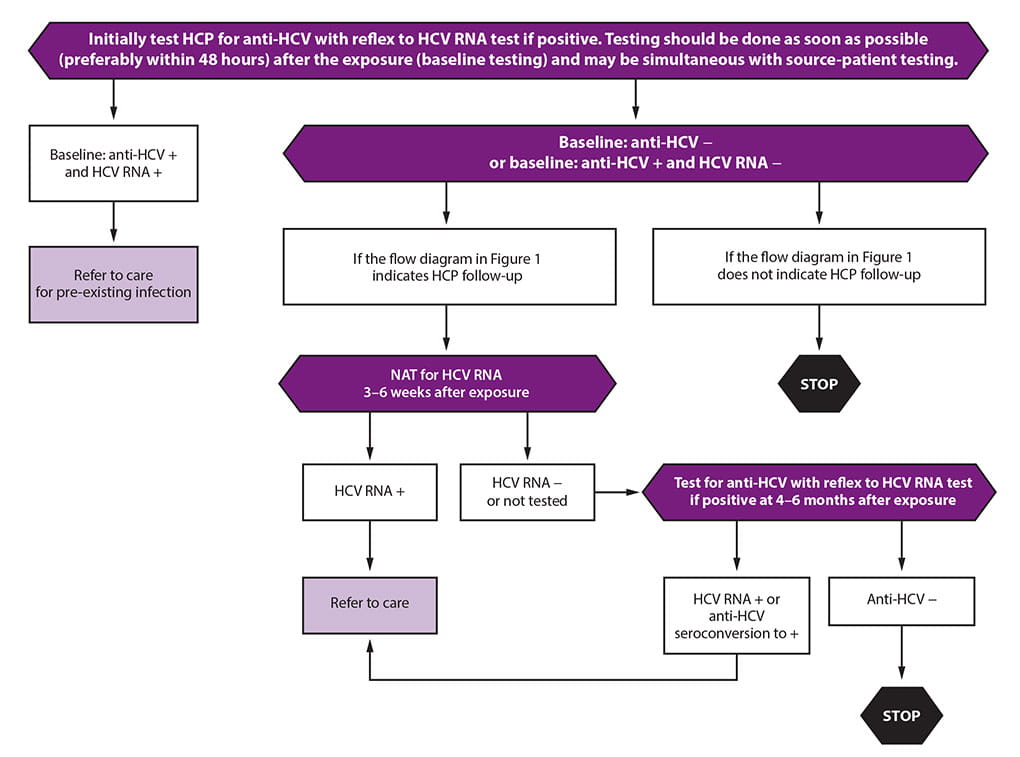 Credit: Image adapted from Moorman AC, de Perio MA, Goldschmidt R, et al. Testing and Clinical Management of Health Care Personnel Potentially Exposed to Hepatitis C Virus—CDC Guidance, United States, 2020. MMWR Recomm Rep 2020;69(No. RR-6):1-8.
Credit: Image adapted from Moorman AC, de Perio MA, Goldschmidt R, et al. Testing and Clinical Management of Health Care Personnel Potentially Exposed to Hepatitis C Virus—CDC Guidance, United States, 2020. MMWR Recomm Rep 2020;69(No. RR-6):1-8.AT THE FOREFRONT OF LIVER DISEASE TREATMENT
Telemedicine plays an important role in HCV treatment. UH uses the technology to reach rural populations who may not seek care on their own.
In cases of chronic disease that require liver transplant, patients often receive HCV-infected livers. University Hospitals has achieved excellent clinical outcomes in such HCV-positive organ transplants. “We’re treating and curing the HCV within 1-3 months after transplant,” Dr. Cohen says.
Early testing and effective treatments will help lower the numbers of HCV infection. Until the virus is eliminated, the latest guidelines will help keep patients and healthcare professionals safe.
To refer a patient with liver disease, call 1-866-UH4-CARE. For updated guidance on HCV diagnosis, strategy, and treatment, read the IDSA guidance document at hcvguidelines.org. You can find the CDC guidance for healthcare professionals at cdc.gov/mmwr/volumes/69/rr/rr6906a1.htm.
References
- Naggie S, Fierer DS, Hughes MD, Kim AY, Luetkemeyer A, Vu V, Roa J, Rwema S, Brainard DM, McHutchison JG, Peters MG, Kiser JJ, Marks KM, Chung RT; Acquired Immunodeficiency Syndrome Clinical Trials Group (ACTG) A5327 Study Team. Ledipasvir/Sofosbuvir for 8 Weeks to Treat Acute Hepatitis C Virus Infections in Men With Human Immunodeficiency Virus Infections: Sofosbuvir-Containing Regimens Without Interferon for Treatment of Acute HCV in HIV-1 Infected Individuals. Clin Infect Dis. 2019 Jul 18;69(3):514-522. doi: 10.1093/cid/ciy913. PMID: 31220220; PMCID: PMC6637278.
- Scott LJ. Ledipasvir/Sofosbuvir: A Review in Chronic Hepatitis C. Drugs. 2018 Feb;78(2):245-256. doi: 10.1007/s40265-018-0864-z. PMID: 29380288.
- FDA approves Vosevi for Hepatitis C. FDA News Release, July 18, 2017.


