UH Continues to Battle COVID-19 Through Research and Clinical Trials
May 18, 2020
By UH Clinical Trials Center for Clinical Trials Awareness Week 2020
The UH Clinical Research Center is championing COVID-19 research efforts by opening the “UH COVID-19 and Coronavirus Biorepository” under the leadership of Vice President of Research and Director, UH Clinical Research Center, Grace McComsey, MD, FIDSA. This protocol will allow investigators access to de-identified data and biological samples to help further COVID-19 research while ensuring privacy of COVID-positive patients.
 Grace McComsey, MD
Grace McComsey, MD Robert Salata, MD
Robert Salata, MDIn addition to the study discussed above, Robert Salata, MD, Chairman, Department of Medicine, Physician-in-Chief, University Hospitals, and infectious specialist is leading the ARMS-1 clinical research study that will enroll approximately 4,300 participants across UH, MetroHealth, and Summa Health systems. This research study aims to stop the spreading of COVID-19 infection to caregivers through administration of an investigational drug via oral spray. Other COVID-19 trials that the UHCRC is supporting are immune modulators, including Tocilizumab, Gimsilumab, CD24Fc, Tofacitinib, and expanded access to Convalescent Plasma.
We also asked for feedback from Principal Investigators (PIs) and study teams supporting COVID-19 research across the system concerning how the pandemic has impacted their work as well as what drives and inspires them.
Arash Rashidi, MD, Director of the Nephro-Oncology Program, at University Hospitals is leading STOP-COVID (Study of the Treatment and Outcomes in Critically-Ill Patients with COVID-19). This multi-center observational study will look at the clinical features and outcomes of critically-ill patients with COVID-19 who are admitted to intensive care units across the U.S.
Dr. Rashidi and his team would like to determine independent risk factors for hospital mortality and acute organ injury, and identify treatment strategies associated with improved survival.
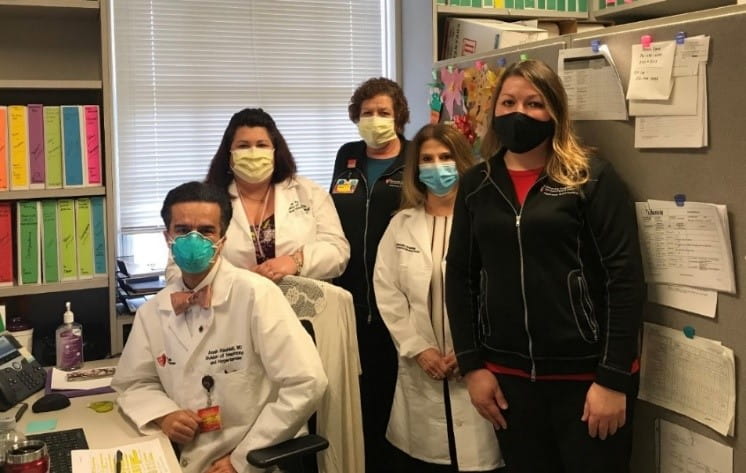 Pictured: (left to right) Arash Rashidi, MD (PI), Tricia Young, RN (Clinical Nurse Research Specialist), Renee Carter (Clinical Research Specialist), Vicki Donley (Clinical Nurse Research Specialist), and Nicole Albert (Clinical Research Regulatory Specialist)
Pictured: (left to right) Arash Rashidi, MD (PI), Tricia Young, RN (Clinical Nurse Research Specialist), Renee Carter (Clinical Research Specialist), Vicki Donley (Clinical Nurse Research Specialist), and Nicole Albert (Clinical Research Regulatory Specialist)“The COVID-19 pandemic has had a devastating impact around the world. Combating this pandemic will require a multidisciplinary approach from the medical research community, including translational studies to understand the pathogenesis of disease, randomized controlled trials of novel and repurposed pharmacotherapies, and rigorously conducted epidemiologic studies that include granular patient-level data,” said Dr. Rashidi, whose team has been working consistently to ensure accurate data.
Colin McCloskey, MD, Emergency Medicine, Anesthesiology-Critical Care, UH, is serving as PI on the ECMOCard study (ExtraCorporeal Membrane Oxygenation for 2019 novel Coronavirus Acute Respiratory Disease). The goal of that study is to describe clinical features, including the severity of pulmonary dysfunction, ECMO technical characteristics, duration of ECMO complications, and survival of patients with COVID-19.
 Colin McCloskey, MD
Colin McCloskey, MD“Given the volume of COVID patients requiring ICU care, there is a large data acquisition burden. Nicely, the EMR has all necessary data elements, so most of the work can be performed remotely from patient care areas,” Dr. McCloskey explained.
“The most fulfilling aspect of this research process is the both the multidisciplinary nature at our institution (Team comprised of Anesthesia Critical Care, Pulmonary Critical Care, Cardiac Surgery) as well as the ability to take part in a study including over 230 centers internationally at time of writing. It’s awesome to collaborate at that scale.”
Olivia Giddings, MD, PhD, Pediatric Pulmonology, Pulmonary and Critical Care Medicine, is serving as PI on two studies. Dr. Giddings said, “These two studies are investigating the use of different drugs to modulate the inflammatory response in patients with COVID-19. The immune response seems to be over-reactive in some patients with COVID-19 and can lead to severe lung disease such as ARDS (Acute Respiratory Distress Syndrome) as well as kidney failure and damage to other organs.”
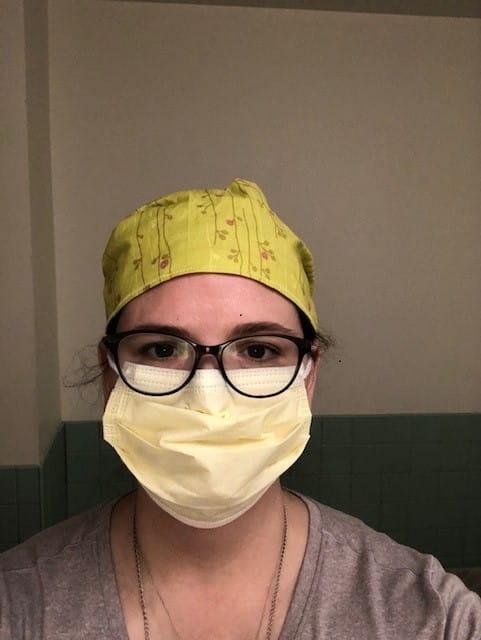 Olivia Giddings, MD, PhD in the COVID-19 ICU
Olivia Giddings, MD, PhD in the COVID-19 ICUManaging two studies during a pandemic is no easy feat, but a strong, collaborative team can make anything happen.
Dr. Giddings exclaimed, “The team has been unbelievable. There has been so much willingness to help, to work extra hours, and for everyone to pitch in—it has been incredibly inspiring.”
”Being involved in the development and testing of therapies that may benefit those patients, and interacting with all of the members of the team that have developed those therapies – from the scientists who developed the drug, to the companies that have seen promise in those therapies and are sponsoring a clinical trial, to the research coordinators, regulatory coordinators, pharmacists and nurses and everyone involved in enrolling patients in a study and gathering the data, and most importantly the patients themselves who are undergoing extra blood draws or extra procedures to hopefully benefit others who get this virus in the future – is an incredibly rewarding experience.”


