Next-Generation Leadless Pacemakers and an Emerging Solution for Heart Failure
June 29, 2021
Three important cardiac electrophysiology trials under way at UH Harrington Heart & Vascular Institute
Innovations in Cardiovascular Medicine & Surgery | Summer 2021
Cardiac electrophysiologists at University Hospitals Harrington Heart & Vascular Institute are offering their patients the latest advanced devices in leadless pacing, as well as cardiac contractility modulation (CCM) therapy, and are enrolling them in three important ongoing clinical trials looking to better define the benefits of the new technologies.
The first trial, Leadless II examining Abbott’s Aveir leadless pacemaker, is enrolling patients with sinus bradycardia with infrequent pauses or unexplained syncope with electrophysiology findings. The trial is also open to patients with chronic atrial fibrillation with 2 or 3° atrioventricular (AV) or bifascicular bundle branch block (BBB). The study is designed to confirm the safety and effectiveness of the Aveir LP System in a subject population indicated for a VVI(R) pacemaker. Primary outcome measures include complication-free rate and pacing thresholds and R-wave amplitudes within the therapeutic range.
 Judith Mackall, MD
Judith Mackall, MD Ivan Cakulev, MD
Ivan Cakulev, MD“The Aveir can only support the ventricular rhythm, so in people who normally have their own heart rate all the time and they just need some support infrequently, it's good to use,” says UH electrophysiologist Judith Mackall, MD, who is leading the Leadless II trial at UH Harrington Heart & Vascular Institute. “What's nice about it that it uses much less energy to pace the heart than typical wires. The battery in these devices is expected to las a relatively long time, usually 12 to 13 years.”
Although the current Leadless II trial does not directly address it, Dr. Mackall says she expects the results will eventually lead to the development of a leadless dual chamber pacemaker.
“They are designing a leadless atrial pacemaker,” she says. “If you had a leadless atrial pacemaker that talks to the leadless ventricular pacemaker, you would then be able to pace and sense in the top chambers and the bottom chambers. You'd be able to keep the heartbeats in sync which is ideal for people who need pacing support and are in normal rhythm. With this Leadless II trial, we're in the beginning phase where we're just putting in the ventricular pacemaker, but we see that this will lead to a dual chamber trial.”
UH Harrington Heart & Vascular Institute has a strong history of conducting clinical trials with electrophysiology devices from Abbott and St. Jude Medical, Dr. Mackall says, dating back almost 30 years.
“We’ve been involved with them on many clinical trials that have really shaped modern technology and modern treatment of heart rhythms,” she says. “They know that we're engaged, that we have many patients who are educated and interested and that we recognize the benefit of getting new technology to patients as soon as possible. In addition, our EP section includes my colleagues, who are like-minded, and nursing staff, who provide exceptional follow-up care.”
Beyond the Leadless II trial, Dr. Mackall is also lead investigator at UH Harrington Heart & Vascular Institute of a post-market clinical trial of Medtronic’s Micra leadless pacemaker. This trial, dubbed Accel AV, is designed to characterize atrioventricular synchrony during rest at one month and three months post-implant in subjects with persistent third degree atrioventricular block (AVB) and normal sinus node function.
Micra’s unique attributes make it ideal for this patient population, Dr. Mackall says.
“They realized that this little Micra unit was able to detect atrial contraction – it detects pressure changes that occur with atrial contraction, is the best way to think about it,” she says. “This device has the ability to listen for intrinsic atrial activity. Therefore, for somebody who has heart block, which means the signals from the top are not making it to the bottom, they can get this device, and it will hear the top and pace the bottom. It’s a leadless single unit that can provide this AV synchrony or synchronized beat in sinus rhythm -- nothing like it out there.”
Dr. Mackall says the post-market Accel AV trial will help address remaining unanswered questions about which patients can benefit most from Micra and how it should be programmed for optimal function. But she says she’s hopeful the ultimate results will provide important insights.
“We are really optimistic about this trial because I think this is providing data that's so relevant to clinical practice,” she says. “Both Leadless II and Accel AV leadless trials are evaluating such innovative technology, It's been such a slow long road to get here. But this is the wave of the future, so we're happy to be on this ride.”
Beyond leadless pacemakers, another cardiac electrophysiology device being studied and offered to heart failure patients at UH Harrington Heart & Vascular Institute is the Optimizer Smart cardiac contractility modulation (CCM) system, produced by Impulse Dynamics and approved by the U.S. Food and Drug Administration in 2019. The post-approval study under way here is enrolling heart failure patients with NYHA functional class III symptoms and reduced (HFrEF) or moderately reduced (HFmrEF) ejection fraction (25-45% inclusive), looking at procedure-related complications, device-related complications, all-cause mortality, change in left ventricular ejection fraction and end-systolic volume, change in BNP and change in QRS duration, as well as change in the patient’s quality of life. This data will be collected at one, two and three years post-device implantation.
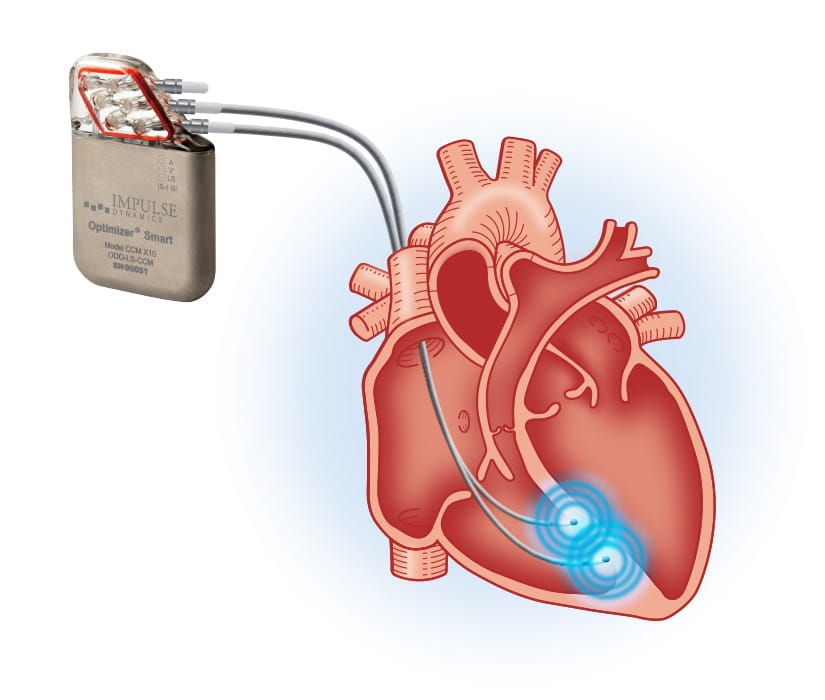 Caption: University Hospitals Harrington Heart & Vascular Institute First in Northeast Ohio to Treat Patient with Heart Failure Using Cardiac Contractility Modulation; First-of-its-kind therapy is intended to improve the contraction of the heart.
Caption: University Hospitals Harrington Heart & Vascular Institute First in Northeast Ohio to Treat Patient with Heart Failure Using Cardiac Contractility Modulation; First-of-its-kind therapy is intended to improve the contraction of the heart.Ivan Cakulev, MD, a cardiac electrophysiologist at UH Harrington Heart & Vascular Institute, is leading the study here. He says he’s happy to be able to provide CCM therapy as an option for heart failure patients – about two -thirds of whom do not qualify for resynchronization therapy. In addition, this is the only approved device that has shown significant benefit in patients with heart failure and higher ejection fractions (> 35%). He also expects to get more rich data on the CCM system as a result of the trial.
“For us, we’re looking number one at side effects and how safe it actually is to do this,” he says. “But we’re also really looking for primary endpoints to be met, like hospitalizations and how much the heart failure improves.”
Dr. Cakulev says he’s encouraged about the opportunity to provide CCM therapy to more heart failure patients.
“The data that led to its approval was fairly robust and very promising – 80 percent reduction in hospitalizations,” he says. “There’s a significant improvement with the vast majority of these patients. We’re dealing with heart failure patients who may have few other options, but now there is this option.”
For more information about these cardiac electrophysiology trials under way at UH Harrington Heart & Vascular Institute or to refer a patient, please call Stacey Mazzurco, RN, Director of Clinical Trials at 216-844-3130.
Contributing Experts:
Judith Mackall, MD
Cardiac electrophysiologist
UH Harrington Heart & Vascular Institute
Irving B. and Virginia Spitz Master Clinician in Cardiology
University Hospitals
Professor
Case Western Reserve University School of Medicine
Ivan Cakulev, MD
Cardiac electrophysiologist
UH Harrington Heart & Vascular Institute
Assistant Professor
Case Western Reserve University School of Medicine


