Medical Device Innovations
Novel Bioresorbable Stent for Congenital Heart Patients
University Hospitals’ researchers are developing a novel Zinc alloy stent for congenital heart disease patients. Endovascular stenting is a mainstay in the care of infants and children with congenital heart disease. Unfortunately, most of the stents currently available to treat narrowed blood vessels cannot grow with young patients as they get bigger. Dr. Martin Bocks and colleagues are developing a novel zinc alloy stent, which can be implanted in young infants and children to treat blood vessel obstruction. This stent will then resorb over time allowing the blood vessel to grow without restriction. Bioresorbable stents would allow several thousand infants each year to avoid the morbidity and mortality of more invasive surgical procedures or the problem of having a stent in place that cannot reach adult size. Using this approach, an appropriately designed family of bioresorbable stents would revolutionize the treatment of congenital heart disease in both infants and older children.

Implantable Pressure Sensor for Fontan Patients
Investigators at University Hospital are working with the team at Integrating Sensing Systems (ISS) to develop a novel miniature wireless implantable pressure sensor specifically for measuring the pressure in the Fontan pathway of patients with single ventricle anatomy. The pressure in the Fontan pathway is arguably the most telling measurement that predicts the overall health of the palliated circulation. The ability to measure Fontan pressures in an ambulatory setting would result in a better understanding of the physiology going on inside a Fontan patient’s body and has the potential to greatly improve the morbidity and mortality associated with this high-risk patient population. Dr. Martin Bocks is currently conducting preclinical studies with the goal of beginning a clinical trial in the next couple of years.
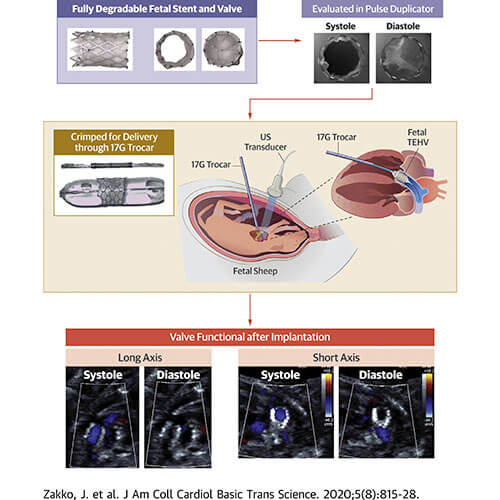
Transcatheter Deployment of Tissue Engineered Heart Valves in the Fetal Lamb
Current valve technology does not allow placement in utero because current valves do not grow and are limited by size of device delivery systems. Drs. Martin Bocks, James Strainic, Aimee Armstrong, and colleagues at UH Rainbow Babies & Children’s Hospital Congenital Heart Program have developed a model for percutaneous implantation of tissue engineered heart valves (TEHVs) in the fetal sheep circulation in order to study neo-valve development in the fetal environment. The primary aim of this study is to assess the feasibility of using this percutaneous technique to perform transpulmonary valve replacement (TPVR) with TEHVs under ultrasound guidance in fetal lambs at 100-110 days gestation, equivalent to 27-28 weeks gestation in human pregnancy.

Bioresorbable ASD Occluder
Atrial Septal Defects (ASDs) are one of the most common types of congenital heart defects, and if left untreated can lead to severe cardiovascular complications. ASDs can often be successfully treated by percutaneous device closure, but the currently available ASD occlusion devices are associated with long-term complications predominantly due to the metal components within them. Dr. Martin Bocks and a team of investigators at UH Rainbow Babies & Children’s Hospital in collaboration with researchers from Georgia Tech propose to develop a new shape memory ASD occlusion device made from a bioresorbable material called poly(glycerol dodecanoate) (PGD). This bioresorbable device will not only allow for complete occlusion but offer the additional benefit of allowing the device material to be resorbed once tissue overgrowth and complete closure has occurred.
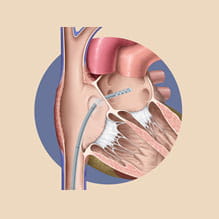
Left Atrial Decompression Catheter for ECMO
Extracorporeal Membrane Oxygenation (ECMO) has become a widely accepted means to provide support for pediatric and adult patients suffering from acute cardiac or pulmonary failure. When severe ventricular dysfunction results in left atrial hypertension while on ECMO, there is a need for left atrial decompression. Dr. Martin Bocks and collaborators at MC3 Cardiopulmonary are developing a specific line of catheters that can be used to decompress the left heart in pediatric patients on ECMO. Supported by a NIH grant, the team has developed prototype catheters that are undergoing final phase of preclinical testing.


