Research Conflict Of Interest (COI)
University Hospitals recognizes that government-sponsored research and industry-sponsored research play a necessary and important role in advancing wellness, healthcare, and medicine, but also recognizes its risk for perceived, potential, or actual conflicts of interest in research. The Human Research Protection Program (HRPP) provides support for all individuals who share responsibility for the design, conduct, or reporting of research and related activities at University Hospitals who may have a potential for a conflict of interest.
If the UH HRPP identifies a potential for an actual, potential, or perceived conflict of interest, the conflict may be managed, reduced, acknowledged, or eliminated at the UH HRPP’s discretion in collaboration with the conflicted investigator. The UH IRB has the final authority to decide whether the conflict of interest and its management, if any, allows the research to be approved.
What Constitutes an Individual Conflict of Interest in Research?
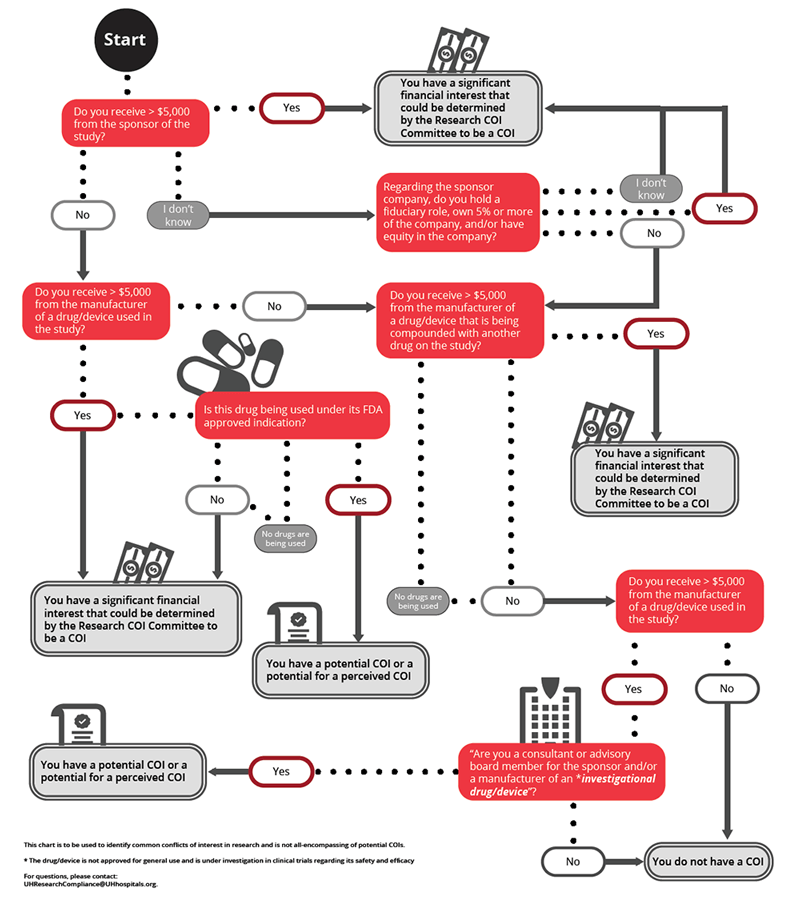
- Any compensation made to the investigator by any sponsor of the covered clinical study in which the value of compensation could be affected by study outcome.
- A proprietary interest in the tested product including, but not limited to, a patent, trademark, copyright or licensing agreement.
- Any equity interest in any sponsor of the covered clinical study, i.e., any ownership interest, stock options, or other financial interest whose value cannot be readily determined through reference to public prices.
- Any equity interest in any sponsor of the covered study if the sponsor is a publicly held company and the interest exceeds $5,000 in value.
- Remuneration from publicly traded entity in the 12 months preceding the disclosure that in aggregate exceeds $5,000 (e.g., salary and consulting income, honoraria, paid authorship, or other payments for services).
- Equity interests such as stocks, stock options, warrants, contractual rights to acquire or receive ownership interests, or other ownership interests in a publicly-traded company that is more than $5,000 or any amount in a non-publicly-traded company in the 12 months preceding the disclosure.
- Remuneration from non-publicly traded entity in the 12 months preceding the disclosure that in aggregate exceeds $5,000 in equity.
- Intellectual property rights and interests (e.g., patents, copyrights), upon receipt of income related to such rights and interests.
- Service as an officer, director, or in any other executive fiduciary position in a related research sponsor whether or not remuneration is received for such service.
- Reimbursed or sponsored travel, except travel paid by U.S. federal, state, or local government agencies or other U.S. institutions of higher education, academic teaching hospitals, or research institutes affiliated with an institution of higher education.
- Any situation the UH HRPP and/or UH IRB determine to be a conflict of interest which has the potential to introduce bias in the research.
I Have an Individual Research COI or Know about a Research Institutional COI, What Should I Do?
Download PDF
Contact Karuna Ramcharran (Karuna.Ramcharran@UHhospitals.org) or Research Compliance (UHResearchCompliance@UHhospitals.org) for next steps on COI management.
UH GPS Education: Identifying & Managing Conflicts of Interest in Research
If you are a UH employee, please ensure you have completed the most recent Relationships of Interest questionnaire in SAI360.


