Institutional Review Board Scope & Responsibilities
The UH Institutional Review Board (IRB) reviews all human subject research protocols that includes UH patients, PHI, or data. In addition, all studies involving human subjects carried out on the premises, or using the services, of any University Hospitals Health System facility must be submitted to the UH IRB.
- Statement of Compliance (PDF)
IRB Policies
The Investigator Manual for IRB Submissions is designed to guide you through policies and procedures related to the conduct of human subjects research specific to the UH IRB. Investigators are required to abide by procedures as described in the manual.
Meeting Dates and Submission Deadlines
The UH IRB consists of three boards, one of which specializes in the review of cancer research. The three committees are referred to as J Committee, M Committee and C Committee.
- The J and M Committees meet every two weeks on Tuesdays (except holidays) at 3:30pm.
- The C Committee meets the 2nd and 4th Thursdays of the month (except holidays) at 2:30pm.
- IRB Meeting Dates
-
- IRB Meeting Dates 2025 (PDF)
- IRB Meeting Dates 2024 (PDF)
- IRB Meeting Dates 2023 (PDF)
- IRB Meeting Dates 2022 (PDF)
Please contact the UH IRB for previous roster versions.
- J Committee Rosters
-
- IRB Roster – J Committee v. 12/22/2025 (PDF)
- IRB Roster – J Committee v. 11/10/2025 (PDF)
- IRB Roster – J Committee v. 09/29/2025 (PDF)
- IRB Roster – J Committee v. 08/7/2025 (PDF)
- IRB Roster – J Committee v. 07/01/2025 (PDF)
- IRB Roster – J Committee v. 04/01/2025 (PDF)
- IRB Roster – J Committee v. 01/15/2025 (PDF)
- IRB Roster – J Committee v. 01/02/2025 (PDF)
- IRB Roster – J Committee v. 8/1/2024 (PDF)
- IRB Roster – J Committee v. 6/14/2024 (PDF)
- IRB Roster – J Committee v. 10/11/2024 (PDF)
- IRB Roster – J Committee v. 11/21/2024 (PDF)
- IRB Roster – J Committee v. 10/11/2024 (PDF)
- IRB Roster – J Committee v. 10/02/2024 (PDF)
- IRB Roster – J Committee v. 09/20/2024 (PDF)
- IRB Roster – J Committee v. 8/1/2024 (PDF)
- IRB Roster – J Committee v. 6/14/2024 (PDF)
- IRB Roster – J Committee v. 10/11/2023 (PDF)
- IRB Roster – J Committee v. 9/21/2023 (PDF)
- IRB Roster – J Committee v. 6/15/2023 (PDF)
- IRB Roster – J Committee v. 3/17/2023 (PDF)
- IRB Roster – J Committee v. 2/15/2023 (PDF)
Please contact the UH IRB for previous roster versions.
- M Committee Rosters
-
- IRB Roster – M Committee v. 12/22/2025 (PDF)
- IRB Roster – M Committee v. 11/10/2025 (PDF)
- IRB Roster – M Committee v. 09/29/2025 (PDF)
- IRB Roster – M Committee v. 08/07/2025 (PDF)
- IRB Roster – M Committee v. 07/01/2025 (PDF)
- IRB Roster – M Committee v. 04/01/2025 (PDF)
- IRB Roster – M Committee v. 01/15/2025 (PDF)
- IRB Roster – M Committee v. 01/02/2025 (PDF)
- IRB Roster – M Committee v. 11/21/2024 (PDF)
- IRB Roster – M Committee v. 10/11/2024 (PDF)
- IRB Roster – M Committee v. 10/02/2024 (PDF)
- IRB Roster – M Committee v. 09/20/2024 (PDF)
- IRB Roster – M Committee v. 8/1/2024 (PDF)
- IRB Roster – M Committee v. 6/14/2024 (PDF)
- IRB Roster – M Committee v. 10/11/2023 (PDF)
- IRB Roster – M Committee v. 9/21/2023 (PDF)
- IRB Roster – M Committee v. 6/15/2023 (PDF)
- IRB Roster – M Committee v. 3/17/2023 (PDF)
- IRB Roster – M Committee v. 2/15/2023 (PDF)
Please contact the UH IRB for previous roster versions.
- C Committee Rosters
-
- IRB Roster – C Committee v. 12/22/2025 (PDF)
- IRB Roster – C Committee v. 11/10/2025 (PDF)
- IRB Roster – C Committee v. 09/29/2025 (PDF)
- IRB Roster – C Committee v. 08/07/2025 (PDF)
- IRB Roster – C Committee v. 07/01/2025 (PDF)
- IRB Roster – C Committee v. 04/01/2025 (PDF)
- IRB Roster – C Committee v. 01/15/2025 (PDF)
- IRB Roster – C Committee v. 01/02/2025 (PDF)
- IRB Roster – C Committee v. 11/21/2024 (PDF)
- IRB Roster – C Committee v. 10/11/2024 (PDF)
- IRB Roster – C Committee v. 10/02/2024 (PDF)
- IRB Roster – C Committee v. 09/20/2024 (PDF)
- IRB Roster – C Committee v. 8/1/2024 (PDF)
- IRB Roster – C Committee v. 6/14/2024 (PDF)
- IRB Roster – C Committee v. 10/11/2023 (PDF)
- IRB Roster – C Committee v. 9/21/2023 (PDF)
- IRB Roster – C Committee v. 6/15/2023 (PDF)
- IRB Roster – C Committee v. 3/17/2023 (PDF)
- IRB Roster – C Committee v. 2/15/2023 (PDF)
Please contact the UH IRB for previous roster versions.
The deadline for protocol submission is 10 days before the meeting date.
IRB Requirements & Process
The following must be completed prior to IRB submission:
- CREC Certification in Human Subjects Protections for all staff listed on the study personnel table
- Investigator Training for all Investigators and non-Investigator staff as part of Clinical Research Orientation
- UH Research Credentialing for all non-UH personnel that will have access to UH PHI, systems, or conducting research on UH facilities.
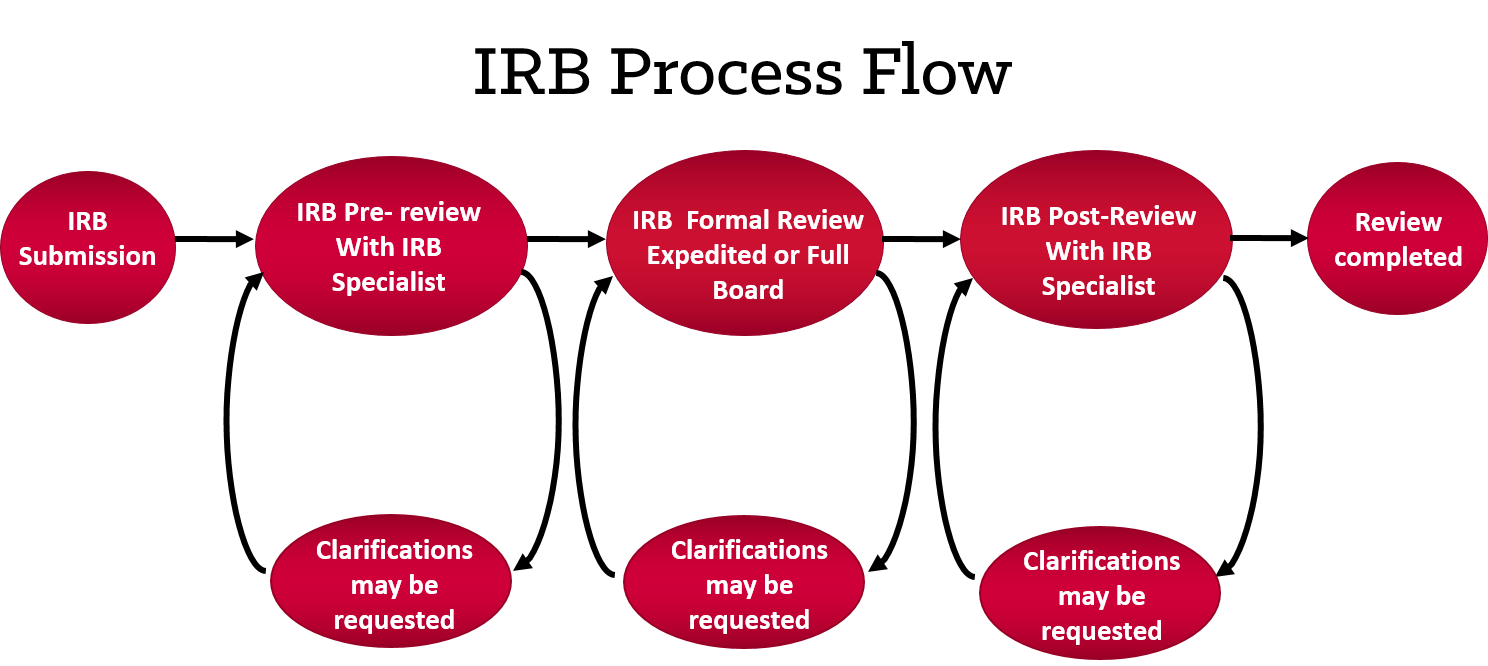
- 1. Submission
-
Study team submits to the IRB via the electronic system. You can login at spartairb.case.edu using your UH or CWRU username and password.
- 2. Pre-Review with IRB Specialist
-
The submission is assigned to an IRB specialist who reviews the entire submission. If clarifications are needed, the IRB specialist will send stipulations back to the study team that should be addressed (this back-and-forth may happen more than once).
- 3. Formal Review
-
Once the study team has sufficiently addressed all stipulations from the pre-review stage, the study will either be:
- Electronically expedited to an IRB chair or vice chair – for not greater than minimal risk studies (with exceptions)
- Assigned to the next full board meeting with an open agenda (meeting agendas are closed and sent out ~six days prior to the meeting date) – for greater than minimal risk studies (with exceptions)
- 4. Post-Review Results
-
The formal review may yield one of five results:
- Approved: No changes are needed and the IRB specialist may send an approval letter immediately.
- Approved with administrative confirmation: The submission is approved pending minor changes. Stipulations will be sent back to the study team and must be addressed before an approval letter may be sent.
- Approved with modifications: The submission is approved pending minor clarifications. Stipulations will be sent back to the study team and must be addressed before an approval letter may be sent.
- Deferred: Not enough information was available for the board to make a determination and the study must be brought back to a subsequent full board meeting. Stipulations will be sent back to the study team and must be addressed before the study is assigned to the next full board meeting for re-review.
- Disapproved: If a study is lacking in scientific merit, if it raises ethical questions that cannot be resolved, or if the risks outweigh the benefits.
- 5. Review Complete
-
After the study is approved it is the responsibility of the PI and the study team to ensure communication with the IRB is maintained.
- Submit a continuing review six to eight weeks before study’s expiration date.
- Submit an addendum if there is any change in the study including (but not limited to): PI changes, protocol changes, informed consent form changes, and application changes.
- Submit personnel change forms to add or remove study personnel.
- Report deviations and adverse events to the IRB in accordance with reporting guidelines outlined in the Investigator Manual for IRB Submissions.
- Submit a closure when you are ready to close the study.
Forms & Templates
New Protocol Submission review requests, including Human Research Determinations, Exemptions, HUDs and Emergency Use requests must be submitted for review in the SpartaIRB System.
In SpartaIRB, much of the information about your study will be submitted to the IRB in a protocol template document that is a Microsoft Word file. When submitting in SpartaIRB, the use of one of these templates is REQUIRED.
- HRP-503BIO: Biomedical Protocol template
- HRP-503DATA: Chart Review Data + Specimens Protocol template
- HRP-503NHR: Non-Human or Non-Research Protocol template
- HRP-503SUPP: Supplemental Form
In addition to the above templates there are several other templates and forms that are required and/or available for use. For example Recruitment Templates and a HIPAA Waiver Forms. All templates can be found in the SpartaIRB System.


